FDA warning letter names' smart e-cigarettes: involving 6 brands including FUME, Craftbox, North Vape, etc
Leave a message
FDA warning letter names' smart e-cigarettes': involving 6 brands including FUME, Craftbox, North Vape, etc
2Firsts found through sorting out the latest batch of warning letters released by the FDA on October 30th that this incident involves six e-cigarette brands, namely FUME, Craftbox, North Vapor, Posh, Halo Vapor, and Swype.
On October 31st, Two Up 2Firsts reported that the US Food and Drug Administration (FDA) has issued warning letters to nine online retailers and one manufacturer, accusing them of selling unauthorized smart e-cigarettes (click to read the article). Two to two firsts found through sorting FDA warning letters that this warning letter involves six e-cigarette brands, namely FUME, Craftbox, North Vapor, Posh, Halo Vapor, and Swype.
Two to two firsts noted that in the FDA's ten warning letters, the FDA described "these product labels or advertisements may attract young people by mimicking smartphones or other smart technologies" and "may attract teenagers by mimicking gaming products", and called these products "very concerning".
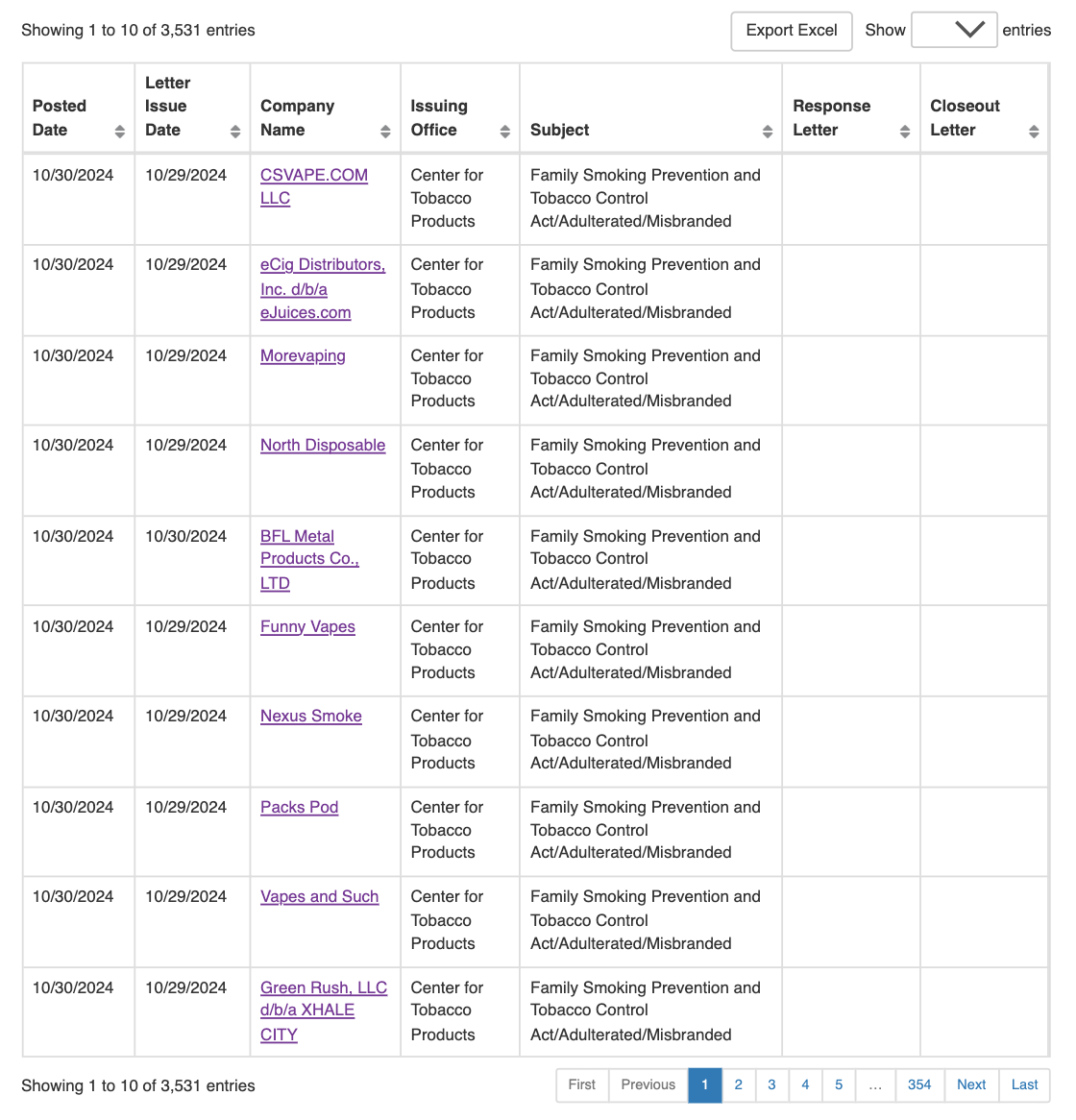
Warning Letter Issued by FDA | Image Source: FDA
The following is a list of brands and their products mentioned in two FDA warning letters compiled by Up to 2Firsts.
FUME
Its product WeFume Vape 30K Puffs was mentioned three times in the warning letter.
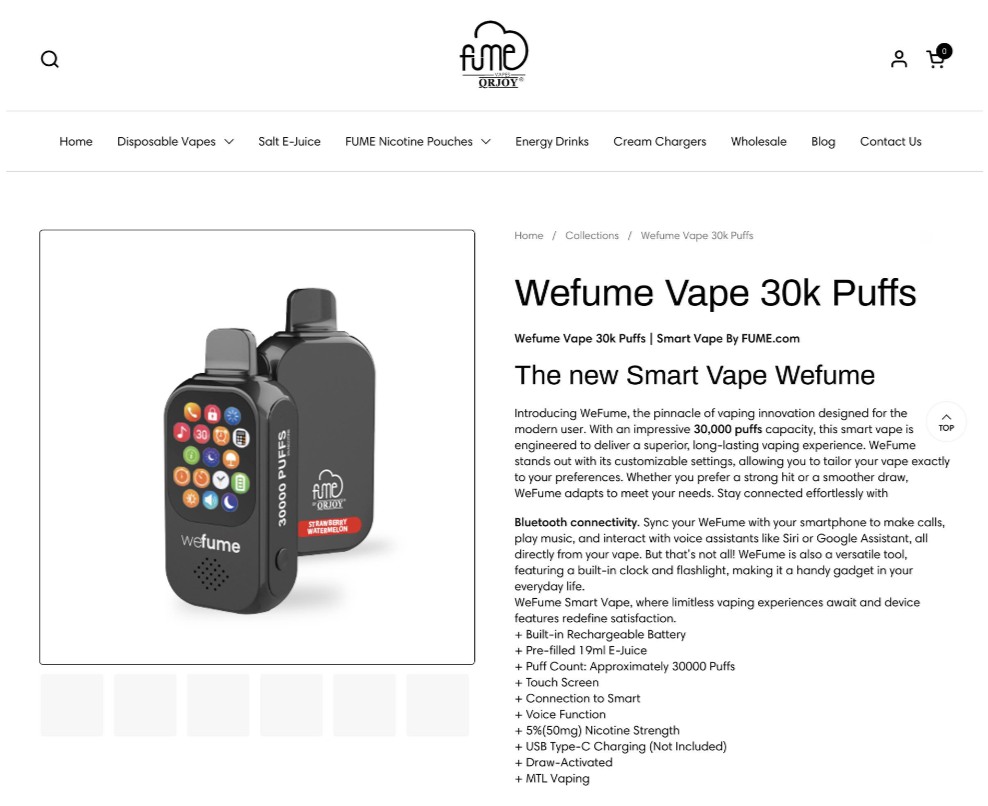
WeFume 30K Puff Image Source: Fume
Craftbox
Craftbox V-Touch 30k Puff Smart disposable electronic cigarette is recommended 4 times in the notification, Craftbox V-Play 20K Puff disposable electronic cigarette is recommended 5 times, Craftbox V-Touch 30000 Puff Phone Call disposable electronic cigarette is recommended 1 time

Image source: FDA
North Vape
Its product North South Connect 35K Puffs Phone Disposable Vape was mentioned twice in the warning letter, South Connect 35K Disposable Vape Bluetooth 900mAh was mentioned once.

Image source: FDA

Image source: FDA
Posh
Their products, Posh Xtron 30000 Disposable Vape and Posh Pro Max 30000, were mentioned once each in the warning letter.

Image source: FDA
Halo Vapor
Its product Halo Vapor Synix 30K was mentioned twice in the warning letter.

Image source: FDA
Swype
Its product Swype 30K Disposable Vape was mentioned once in the warning letter.

Image source: Dealer website vapezilla
According to FDA information disclosure, when the FDA discovers what it deems to be a serious violation of federal requirements, it will notify the relevant parties.
This notification usually takes the form of a warning letter. Warning letters will point out issues such as poor production practices, issues with product efficacy promotion, or incorrect usage instructions. This letter provides an opportunity for companies or individuals to address FDA issues and requests a response within a certain period of time. The response may include a corrective plan, and then the FDA will inspect to ensure that corrective measures are sufficient.
If a company or individual disagrees with the FDA's opinion, they have the opportunity to provide the FDA with their reasons and supporting information. These communications and other actions between the FDA and the recipient of the letter may alter the regulatory status of the issues discussed in the letter.
As of October 30, 2024, the FDA has approved 34 electronic cigarette products and devices. The agency maintains a printable single page flyer that lists all authorized e-cigarette products, which retailers can review to determine which products can be legally marketed and sold in the United States. Entities that manufacture, import, sell, or distribute electronic cigarettes without necessary pre-market authorization will face enforcement risks.







